Peptide drugs and protein drugs, compared to traditional small molecule drugs, has the following disadvantages:
- Injection is a common route of administration for peptide drugs. The invasive nature lowers the patient compliance.
- Most peptides and protein drugs have short circulation time and thus short half-lives. They are prone to intracellular and extracellular degradation, and renal elimination.
- Higher immunogenicity due to large sizes.
- Low permeability.
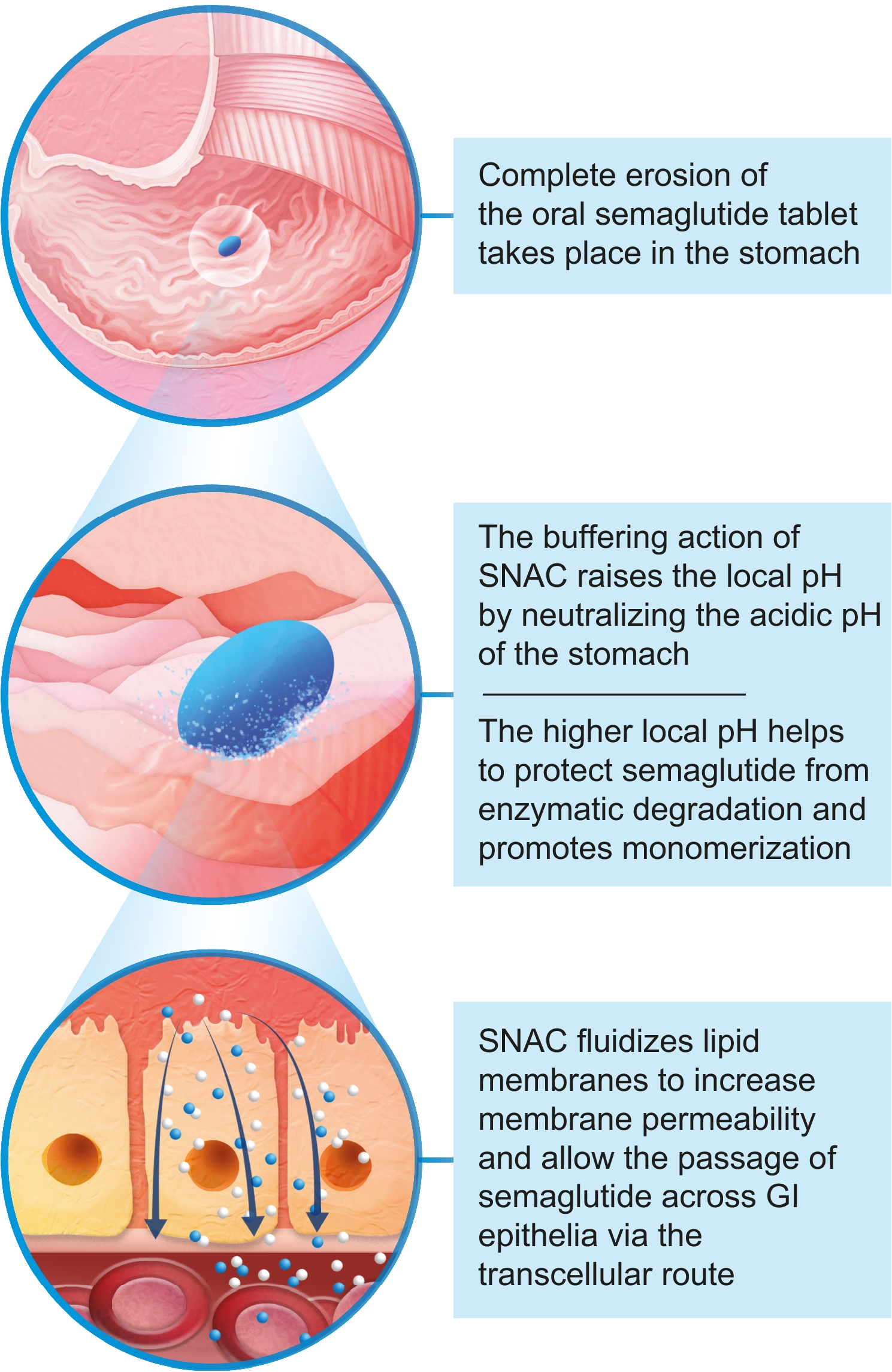
For peptide drugs, one development trend is to optimize the drug delivery from the unpleasant injection to oral ingestion. Apart from covalent modifcation such as lipidation seen in approved peptide drugs such as Semaglutide, a class of excipients known as absorption promotor also draws a great attention.
About SNAC
Salcaprozate sodium (SNAC, CAS No. 203787-91-1), first developed by Emisphere in the 1990s, is an oral absorption promoter / permeation enhancer. SNAC is a synthetic N-acetylated amino acid derivatives of salicylic acid, and can be used to overcome the challenges of oral pepetide delivery.

The molecular structure of SNAC