Market potential of radiopharma & RDCs
RDCs are currently being developed for cancer. According to the WHO IARC, in 2022, the five most common cancers resulted in 19,956,054 new cases and 9,736,520 deaths. It is estimated that by 2050, the incidence of all cancers will reach 35.3 million. Consequently, the spending on cancer is expected to rise. In terms of radiopharmaceuticals, the global market is estimated to be 28.5 billion USD in 2026 from 18.2 billion USD in 2021, at a CAGR of 9.4% (2021-2026), according to BCC Research.

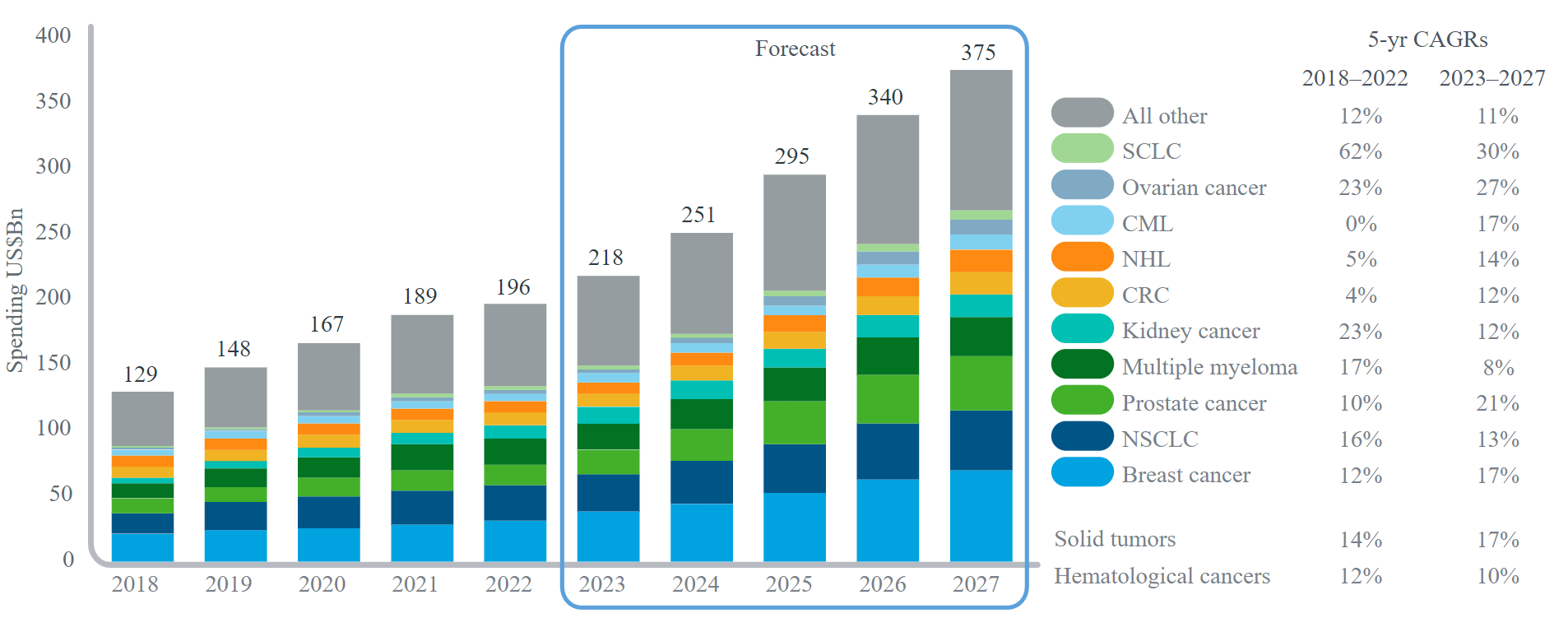
src: IQVIA Oncology Link, Apr 2023
Prostate cancer is the MOST COMMON cancer in men, and prostate-specific membrane antigen (PSMA) is the RDCs' main pipeline target (the 5-year CAGR of 2023-2027 for the medicine spending on prostate cancer is estimated to be 21%). PSMA is a transmembrane protein normally found in small amounts in the prostate gland. However, in cancerous situations, the level of PSMA expressed is markedly higher, and the expression level correlates with the aggressiveness of the disease, which makes PSMA an ideal prostate cancer target.

src: Hyväkkä, A. et al. More Than Meets the Eye: Scientific Rationale behind Molecular Imaging and Therapeutic Targeting of Prostate-Specific Membrane Antigen (PSMA) in Metastatic Prostate Cancer and Beyond. Cancers 13, 2244 (2021).
In addition, radiopharmaceuticals and RDCs are frequently used in PET/CT and MRIs. Particularly, the RDCs approved by the FDA are primarily used for PET imaging purposes; thus, the market for RDCs is directly linked to the accessibility of PET equipment. On a global scale, there is notable inequity in the availability of PET equipment. According to the IMAGINE database from by IAEA, high-income countries, on average, have access to 3.522 PET scanners per 1 million people. This is followed by 0.301 scanners for upper-middle-income countries, 0.155 for lower-middle-income countries, and a mere 0.004 for low-income countries.
Given the rising trend in both cancer incidence and the potential accessibility of PET scanners across the globe, it can be deduced that the market growth potential for RDCs will continue to rise.

src: IMAGINE - PET scanners (per 1 mil)
A throwback to the FDA approvals
Novartis is undoubtedly a key player in the radiopharmaceutical and RDCs field. To provide an overview of the market competition, the following paragraphs will detail all FDA-approved radiopharmaceuticals and RDCs since 2016.


approved in 2023-05 by the FDA
developed & marketed by Blue Earth Diagnostics
Flotufolastat F-18 (18F-rhPSMA-7.3) is an 18F-labeled ligand that targets the PSMA. It is used for PET imaging in men with prostate cancer who have suspected metastasis or recurrence. Compared to other diastereoisomers, flotufolastat F-18 has a faster clearance from the blood pool, liver, and kidney, and a high level of accumulation in tumors. It has a longer half-life and can be produced in larger batches.


approved in 2022-03 by the FDA
developed by Radiomedix Inc; marketed by Novartis
Lutetium Lu-177 vipivotide tetraxetan (Lutetium (177Lu) vipivotide tetraxetan) is a radioligand therapeutic agent used for the treatment of PSMA–positive metastatic castration-resistant prostate cancer. It consists of a radionuclide (177Lu) linked to a moiety that specifically binds to PSMA, a transmembrane protein expressed in prostate cancer. It is effective in targeting PSMA and has shown promise in treating prostate cancer.

first approved in 2020-12 by the FDA
marketed by UCLA & UCSF

approved in 2021-12 by the FDA
marketed by Telix

approved in 2022-03 by the FDA
marketed by Novartis
The FDA NDA approval of the pioneering drug for PET imaging of PSMA-positive lesions in prostate cancer was the result of a collaborative academic initiative by UCLA and UCSF. 68Ga-PSMA-11 was the first drug for PET imaging of PSMA-positive lesions in men with prostate cancer. Initially, it was only accessible through UCLA and UCSF. The market exclusivities were waived due to a unique regulatory approach for the NDA submission; therefore, the PET imaging providers can submit ANDAs immediately. Then in December 2021 and March 2022, two brand-name drugs were approved by the FDA.


approved in 2021-05 by the FDA
marketed by Progenics Pharmaceuticals, Inc.
Piflufolastat F18, also known as [F-18]-DCFPyL, is a urea-based radiopharmaceutical (18F) that binds to PSMA, allowing for the visualization of cancerous prostate tissue through PET imaging. It was approved by the FDA in May 2021 under the brand name Pylarify, and by the EMA in July 2023 under the brand name PYLCLARI for primary staging or localizing recurrence of prostate cancer.


approved in 2020-09 by the FDA
developed and marketed by RadioMedix and Curium
Copper Cu-64 dotatate is a newly approved Cu-labeled (64Cu) somatostatin analog for PET imaging for neuroendocrine tumors. It has advantages over 68Ga-labeled somatostatin analogs, including a longer half-life, once-a-day production, and lower positron energy for improved spatial resolution. Further studies are needed to compare Copper Cu-64 dotatate with other tracers. PET using Copper Cu-64 dotatate outperforms single-photon emission computed tomography (SPECT) using 111In-DTPA-octreotide, detecting twice as many lesions. The target of this drug is SSTR.

approved in 2019-08 by the FDA
marketed by UIHC PET Imaging
Edotreotide gallium Ga-68, also known as Ga-68-DOTATOC, is an 8 amino acid peptide bound to the chelator DOTA, which coordinates with 68Ga. It is used for PET to localize neuroendocrine tumors that are positive for SSTR.


approved in 2018-01 by the FDA
developed and marketed by Advanced Accelerator Applications (Novartis)
A radiopharmaceutical agent approved for the treatment of gastroenteropancreatic neuroendocrine tumors. Lutetium Lu 177 dotatate displays a higher uptake of radioactivity (177Lu) in tumors and better residence times compared to alternative somatostatin analogs. The target is SSTR, and it has shown a lower whole-body retention, indicating a potentially lower risk for bone marrow toxicity.

approved in 2016-06 by the FDA
developed and marketed by Advanced Accelerator Applications (Novartis)
Dotatate gallium (68Ga) is a radiolabeled somatostatin-2 receptor analog used for PET imaging of SSTR-positive neuroendocrine tumors. It has a high affinity for somatostatin-2 receptors and is rapidly excreted from non-target sites, making it an ideal candidate for imaging these tumors. Ga-68 dotatate exploits its ability to detect somatostatin receptor scintigraphy, which has a high diagnostic value that tends to change with tumor grade.
References
https://www.iarc.who.int/infographics/global-cancer-burden-growing-amidst-mounting-need-for-services/
https://www.bccresearch.com/market-research/healthcare/radiotherapy-technologies-markets-report.html
https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/global-oncology-trends-2023
https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINEMaps4.html
Hyväkkä, A. et al. More Than Meets the Eye: Scientific Rationale behind Molecular Imaging and Therapeutic Targeting of Prostate-Specific Membrane Antigen (PSMA) in Metastatic Prostate Cancer and Beyond. Cancers 13, 2244 (2021).