Introduction
In a previous article, we discussed when to consider using flow chemistry over conventional batch processes for chemical synthesis. One compelling example highlighting the advantages of flow chemistry comes from the manufacturing route development for belzutifan, a recently approved drug by Merck for treating certain types of renal cell carcinoma. Belzutifan is a first-in-class hypoxia-inducible factor-2 alpha inhibitor therapy approved in the United States in August 2021. Belzutifan holds the distinction of being the first drug to be awarded an "innovation passport" from the UK Medicines and Healthcare products Regulatory Agency (MHRA), underscoring its significance in the field of oncology.
Bottecchia et al. have made a compelling case for the adoption of flow chemistry in the synthesis of this remarkable drug. Based on the article titled Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination, we will delve into their rationale and explore the advantages they have uncovered through their research.
Why Flow Chemistry + Photochemistry for Belzutifan?
First, let's discuss the synthesis route of Belizutifan from an intermediate with CAS No. 1672665-55-2. A spiro ring is formed, which then undergoes a benzylic bromination. The resulting benzylic bromide (CAS No. 2739737-08-5) is further oxidized and then reduced with Noyori's Catalyst, yielding another Belizutifan intermediate (CAS No. 2738675-96-0). Finally, CAS No. 2738675-96-0 undergoes subsequent fluorination and deoxyfluorination, giving Belzutifan API.

Synthesis route overview of Belzutifan
The synthesis of Belzutifan involves a key transformation: a benzylic bromination step (from the Belzutifan intermediate CAS No. 1672665-53-0 to the Belzutifan intermediate CAS No. 2739737-08-5). In their clinical supply route, the authors initially employed a thermal radical process, which proved to be sensitive to temperature fluctuations and prone to the formation of undesirable byproducts. A temperature of 40 degC was required to initiate the radical mechanism through a radical initiator AIBN, which is a hazardous substance but would impact the stability of the product that was prone for overbromination and deketalization. Such challenges not only posed potential batch failure risks but also raised safety and environmental concerns.

src: labchem-wako

Structure of 2,2'-Azobis(2-methylpropionitrile), AIBN, CAS No. 78-67-1
Recognizing the need for a more robust and sustainable approach, the authors turned to flow chemistry, specifically a continuous flow visible-light-induced benzylic bromination process that uses light as the radical initiator. This photochemical method circumvented the need for azo radical initiators (i.e. AIBN in this case) and proceeded at room temperature, offering improved safety and robustness compared to the alternative thermal process.

The comparsion of the thermal and photochemical processes en route to Belzutifan API production.
src: Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).
Advantages Unveiled Through Studies
To validate their approach, the authors conducted a series of studies, exploring various aspects of the flow chemistry process. Here are some key findings that highlight the advantages of their chosen methodology:
Reaction Optimization and Control
Through in situ LED-NMR spectroscopy experiments, the authors gained valuable insights into the reaction mechanism and selectivity. This understanding enabled them to optimize reaction conditions, such as the use of substoichiometric citric acid as an additive to enhance reaction rates and mitigate the impact of copper impurities.
Br2 Generation and Irradiation Time: Key Factors in Synthesis Process Control
The reaction profile collected below showed an induction period, consistent with the hypothesis that formation of Br2 from DBDMH is necessary before the reaction can proceed. Past full conversion, the desired product 2 can undergo further radical bromination to yield the undesired gem-dibromo compound 3. In an experiment where light irradiation was temporally modulated between on and off states, the reaction stopped in the absence of light, confirming the photoinduced nature of this transformation.

In situ LED−NMR spectroscopy experiments. In both panels, the reaction was performed with starting material 1 (30 mM) and 1.05 equiv of DBDMH in CD3CN. Abbr: SM = starting material; Pdt = product.
src: Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).
Crucially, when a catalytic quantity of Br2 (5 mol %) was added to a 200 mg scale batch reaction in the presence of DBDMH, the induction period disappeared. This observation supported the hypothesis that Br2 is the photoactive species in the system, and its generation from DBDMH is the source of the induction period. Overbromination to yield byproduct 3 was also identified as a light-catalyzed process, the impact of which could be minimized by appropriate control of the irradiation time.
Furthermore, fate and purge studies in downstream processing established the need to run this process at consistent conversion levels and control the amount of gem-dibromo compound 3. The decision to specifically control the amount of 3 was driven by its poor stability – upon degradation, this side product eliminates HBr and catalyzes the deprotection of the ketal in the desired product 2.
These considerations highlighted the importance of targeting an ideal conversion window to achieve process robustness. The necessity to tightly control both the reaction and irradiation times, coupled with the practical aspects of photochemistry on scale, pointed the authors toward the use of a flow reactor to perform this transformation, allowing precise control over these parameters.
Mitigating Copper Impurities in the Synthesis Process
The authors also identified copper impurities as a significant factor impacting the reaction rate. Subjecting a lot of starting material 1 contaminated with 22 ppm Cu to irradiation in the presence of DBDMH required a reaction time of 20 minutes to reach full conversion, as opposed to 3 minutes in the absence of Cu. Spiking experiments with increasing amounts of CuBr confirmed a direct correlation between the Cu content and the reaction rate. While upstream controls minimized residual copper levels, the dramatic influence at such low levels necessitated further mitigation.
Accordingly, the authors screened known copper chelators as potential additives. EDTA and nitrilotriacetic acid (NTA) emerged as feasible additives to restore an initial reaction rate comparable to the absence of Cu. Further screening identified citric acid and L-tartaric acid as inexpensive and readily available alternatives. Notably, citric acid surpassed NTA's positive effect on the reaction rate and increased the absorbance of the reaction mixture at 450 nm even before irradiation, suggesting it favored Br2 generation from DBDMH. The increased initial reaction rate and disappearance of the induction period in the presence of citric acid corroborated this hypothesis.

Impact of copper residuals in the photochemical reaction.
src: Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).
Scalability and Reproducibility
By employing photon stoichiometry as a scaling factor, the authors demonstrated the ability to translate their photochemical process from lab scale to multikilogram scale while maintaining consistent performance. The precise control over irradiation and temperature, coupled with the narrow residence time distribution afforded by continuous flow reactors, ensured high conversion and minimized byproduct formation.
A key factor enabling the scalability and reproducibility of this photochemical process was the use of photon equivalents as a scaling parameter. Previous work by the authors' group had shown that the number of photon equivalents can serve as an effective scaling factor to predict photochemical reaction performance across scales and reactors. As long as a given reaction operates under a photon-limited regime and efficient heat and mass transfer are ensured, the number of photon equivalents necessary to drive the reaction to full conversion remains the same regardless of reactor geometry or photon flux.
This principle was demonstrated by comparing reaction profiles obtained in a 1 L batch reactor irradiated at different light intensities (64 or 120 W output). While plotting product yields versus time showed poor overlap due to differing photon fluxes, plotting them as a function of photon equivalents emitted by the light source resulted in good profile overlap. In both cases, delivering 0.8 photon equivalents to the reactor resulted in the same 90% product yield, although the times required differed (7 min at 64 W vs 3.5 min at 120 W).

Light intensity's impact on conversion rate in batch and flow
src: Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).
The use of citric acid additive further improved productive photon capture by increasing Br2 concentration early in the reaction, maximizing absorption of photons delivered by the light source. This focus on photon capture became especially relevant when using a continuous plug flow reactor (PFR) for scale-up, as PFRs typically employ small-diameter tubing with short path lengths. The tight control over irradiation time and narrow residence time distribution in PFRs proved instrumental in ensuring high conversion and minimal formation of the gem-dibromo impurity 3, meeting process purity specifications.
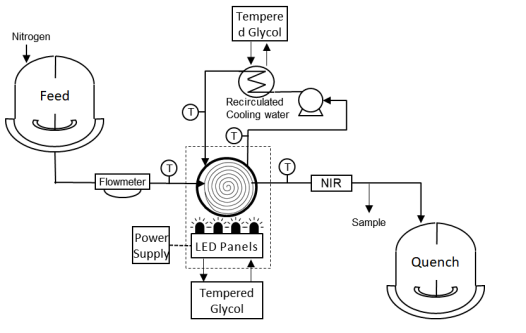
To optimize photon absorption, the authors constructed a small-footprint tubular PFR (8 mm i.d., 890 mL volume) housed in a 20.8 L glass aquarium tank and irradiated with two LED panels of high-intensity blue LED chips. Using this setup and a stop-flow approach to minimize material requirements, they investigated photon stoichiometry and light intensity impacts. Based on 1 L batch results, they anticipated the same photon equivalents would achieve the desired product yield. At 150 and 300 W outputs, 0.8 photon equivalents corresponded to reaction times of 3 and 1.5 min, respectively, and indeed resulted in 88% product yield in both cases. The highest 89% yield was achieved with 1 photon equivalent at both intensities, aligning with batch results.

The Lab setup
src: Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).
Scaling further, the authors demonstrated kilogram-scale synthesis in their kilo-lab facility. Feeding a solution of starting material 1, DBDMH, and 5 mol % citric acid into the PFR via a peristaltic pump, they converted 3.25 kg of 1 to the desired product 2 in approximately 6 hours using a 3.75 min residence time and 2 x 500 W total power input. The 94% assay yield contained only 0.22% of 1 and 2.2% of the gem-dibromo impurity 3. The quenched reaction stream was flowed into a vessel with 2,6-lutidine and 1,3-dimethoxybenzene for downstream processing, maintaining temperature control via cooling throughout the run.
Productivity and Efficiency
Through the development of a second-generation tubular plug flow reactor, the authors achieved a productivity exceeding 100 kg/day for the desired intermediate. This level of productivity, combined with the inherent advantages of flow chemistry, such as improved heat and mass transfer, positions the process as a viable and sustainable option for commercial manufacturing.
Achieving productivity comparable to the alternative thermal radical process for producing the desired product 2 was an essential requirement for implementing this photochemical approach on a manufacturing scale. However, attaining productivities in the range of hundreds of kilograms per day with a continuous flow photochemical reactor has traditionally represented a significant challenge. The authors' initial PFR system achieved a productivity of only 17 kg/day.
To match the required productivity for this key intermediate and build a system operable in a manufacturing environment, the authors focused on developing a second-generation tubular PFR. This new reactor design consisted of a 0.83 L tubular reactor (7.1 mm i.d.) coiled in a spiral fashion and housed between two glass plates forming an enclosure. This enclosure allowed for recirculation of cooling fluid around the reactor tubing. High-intensity LED chips were mounted on a separate metal enclosure surrounding the reactor unit.
Using this reactor assembly in their pilot-plant facility, the authors converted 50 kg of starting material 1 to the desired product 2 in 32 hours of processing time, bringing the system's productivity to 38 kg/day. The results were similar to the preparatory lab scale-up, with 0.09% remaining 1, 2.5% of the gem-dibromo impurity 3, and a 91% yield of product 2 (93% assay yield). Excellent conversion stability was observed throughout the 1.5 min residence time run at 800 W total power input, with consistent temperature and LED performance maintained.

Pilot plant setup
src: Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).
Based on the improved productivity of this pilot-plant reactor, the authors estimated that numbering up by connecting multiple reactor units in series would allow them to attain the required productivity level. They proceeded to build a GMP-qualified reactor train meeting all plant operation safety requirements. This optimized setup employed the same reactor design, with each unit irradiated by two LED panels and an inline quench introduced to quench the reaction stream exiting the final photoreactor unit.
With this production-scale system, the authors achieved a productivity exceeding 100 kg/day, with 0.03% remaining 1, 2.0% of impurity 3, and a 91% yield of product 2 (94% assay yield) at a 3 min residence time. Multiple successful scale-up campaigns followed, and overall, more than 1 ton of the desired product 2 was produced using this photochemical setup and successfully carried through to the subsequent oxidation step.
Notably, the employment of these new photochemical reactors provided a long-awaited solution to the challenge of translating bench-scale photochemistry to a robust manufacturing setting. This breakthrough paves the way for broader adoption of photochemical processes in pharmaceutical manufacturing.
Conclusion
In this work, the authors developed a photochemical benzylic bromination process for the synthesis of the pharmaceutical ingredient belzutifan (MK-6482). Compared to the previous thermal radical approach, the photoflow method offered improved safety and robustness. Mechanistic insights gained from in situ LED-NMR studies guided reaction optimization, including the use of substoichiometric citric acid to enhance the reaction rate, decrease the induction period, and alleviate lot-to-lot variability from copper impurities.
Employing photon stoichiometry enabled predictive scaling across different light intensities and reactor setups. Implementation in a continuous tubular plug flow reactor provided excellent control over irradiation, temperature, and residence time, affording high selectivity for the desired product even at multikilogram scale.
Culminating in the design of a GMP-qualified manufacturing photoreactor train, this work established an elegant solution for transitioning photochemical processes to robust commercial manufacturing. With over 1 ton of product synthesized via this photochemical approach, the authors demonstrated the viability of leveraging the recent "photochemical renaissance" for API production.
This breakthrough paves the way for broader adoption of photochemical methods in pharmaceutical manufacturing, realizing the technology's potential for developing greener and more sustainable processes.
Promoting Safe Production and Green Chemistry
At Unibest, we have been actively investing in flow chemistry to promote safe production and green chemistry principles. We recognize the transformative potential of this technology in addressing the sustainability challenges faced by the pharmaceutical industry.
If you are a pharmaceutical company seeking green chemistry solutions for new product procurement or seeking a sustainable supplier, let's get in touch and we would be delighted to assist you. Our expertise in flow chemistry and our commitment to sustainable practices position us as a valuable partner in your journey towards more efficient and environmentally responsible manufacturing processes.

An industrial-scale flow chemistry equipment for nitrification reactions from Unibest Group
References
Bottecchia, C. et al. Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 26, 516–524 (2022).

















![CAS No. 1672665-55-2, Benzonitrile, 3-[[2,3-dihydro-7-(methylsulfonyl)-1-oxo-1H-inden-4-yl]oxy]-5-fluoro-](http://imrorwxhnlprli5q.ldycdn.com/cloud/lqBpqKkrllSRlknknomliq/1672665-300-300.gif)
![CAS No. 2738675-96-0, Benzonitrile, 3-[[(3R)-2,3-dihydro-3-hydroxy-7-(methylsulfonyl)-1-oxo-1H-inden-4-yl]oxy]-5-fluoro-](http://imrorwxhnlprli5q.ldycdn.com/cloud/lkBpqKkrllSRlknkrolrio/2738675-300-300.gif)


