Patent and Exclusivity Expirations Report
Report generated for the week of 2025-01-06 by Unibest Digital Center. Current analysis scope only include the US FDA.




Summary of Expirations
This week, there are 8 drugs in the patent and exclusivity list. They are:
- AZURITY PHARMACEUTICALS INC's EDARBI, containing active ingredient AZILSARTAN KAMEDOXOMIL
- ABBVIE INC's TRILIPIX, containing active ingredient CHOLINE FENOFIBRATE
- AZURITY PHARMACEUTICALS INC's EDARBYCLOR, containing active ingredient AZILSARTAN KAMEDOXOMIL; CHLORTHALIDONE
- TEVA NEUROSCIENCE INC's UZEDY, containing active ingredient RISPERIDONE
- TEVA PHARMACEUTICAL INDUSTRIES LTD's AIRDUO RESPICLICK, containing active ingredient FLUTICASONE PROPIONATE; SALMETEROL XINAFOATE
- BLUEPRINT MEDICINES CORP's AYVAKIT, containing active ingredient AVAPRITINIB
- BOEHRINGER INGELHEIM's GILOTRIF, containing active ingredient AFATINIB DIMALEATE
- CEPHALON INC's TRISENOX, containing active ingredient ARSENIC TRIOXIDE
Patents Expiring This Week
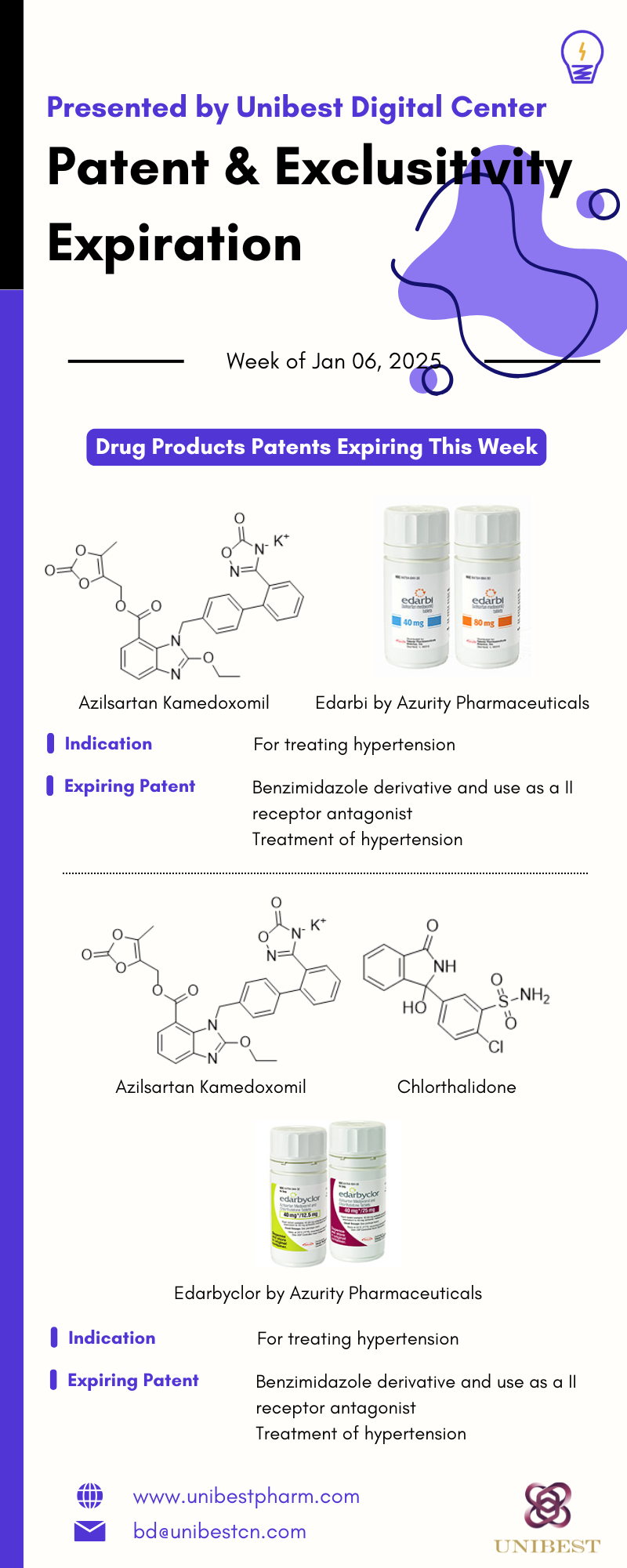
AZILSARTAN KAMEDOXOMIL - TABLET;ORAL - EDARBI
From AZURITY PHARMACEUTICALS INC; for treating hypertension

EQ 40MG MEDOXOMIL; EQ 80MG MEDOXOMIL
Approved in Feb 25, 2011, used as Reference Listed Drug
There are 2 future patent(s) for this application. The earliest expires on 2025-05-22, and the latest expires on 2028-03-26.
| Patent No | Patent Use Code | Patent Use Definition | Patent Expiration Date | Patent Title |
| 7572920 | U-3 | TREATMENT OF HYPERTENSION | 2025-01-07 | Benzimidazole derivative and use as a II receptor antagon |
AZILSARTAN KAMEDOXOMIL; CHLORTHALIDONE - TABLET;ORAL - EDARBYCLOR
From AZURITY PHARMACEUTICALS INC; for treating hypertension


EQ 40MG MEDOXOMIL;12.5MG
EQ 40MG MEDOXOMIL;25MG
Approved in Dec 20, 2011, used as Reference Listed Drug
There are 4 future patent(s) for this application. The earliest expires on 2025-05-22, and the latest expires on 2031-07-01.
| Patent No | Patent Use Code | Patent Use Definition | Patent Expiration Date | Patent Title |
| 7572920 | U-3 | TREATMENT OF HYPERTENSION | 2025-01-07 | Benzimidazole derivative and use as a II receptor antagon |
CHOLINE FENOFIBRATE - CAPSULE, DELAYED RELEASE;ORAL - TRILIPIX
From ABBVIE INC; for treating high levels of fat or cholesterol in the blood

EQ 135MG FENOFIBRIC ACID
Approved in Dec 15, 2008, used as Reference Listed Drug and Reference Standard
There are no future patents for this application.
| Patent No | Patent Expiration Date | Patent Title |
| 7259186 | 2025-01-07 | Salts of fenofibric acid and pharmaceutical formulations thereof |
EQ 45MG FENOFIBRIC ACID
Approved in Dec 15, 2008, used as Reference Listed Drug
There are no future patents for this application.
| Patent No | Patent Expiration Date | Patent Title |
| 7259186 | 2025-01-07 | Salts of fenofibric acid and pharmaceutical formulations thereof |
RISPERIDONE - SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS - UZEDY
From TEVA NEUROSCIENCE INC; for treating a number of mental health disorders including schizophrenia, bipolar mania, psychosis, or as an adjunct in severe depression

100MG/0.28ML (100MG/0.28ML); 125MG/0.35ML (125MG/0.35ML); 150MG/0.42ML (150MG/0.42ML); 200MG/0.56ML (200MG/0.56ML); 250MG/0.7ML (250MG/0.7ML); 50MG/0.14ML (50MG/0.14ML); 75MG/0.21ML (75MG/0.21ML)
All approved in Apr 28, 2023, used as Reference Listed Drug
There are 4 future patent(s) for this application. The earliest expires on 2027-11-12, and the latest expires on 2040-09-11.
| Patent No | Patent Use Code | Patent Use Definition | Patent Expiration Date | Patent Title |
| 10736965 |
|
| 2025-01-12 | Risperidone biodegradable implant |
| 9439905 | U-543 | TREATMENT OF SCHIZOPHRENIA | 2025-01-12 | Risperidone-containing implants and methods of use thereof |
| 9895447 |
|
| 2025-01-12 | Drug-containing PLA implants and methods of use thereof |
| 9925268 |
|
| 2025-01-12 | Drug-containing implants and methods of use thereof |
| 9717799 |
|
| 2025-01-12 | Drug-containing implants and methods of use thereof |
| 8802127 |
|
| 2025-01-12 | Risperidone-containing PLA:PGA implants and methods of use thereof |
Exclusivities Expiring This Week
AFATINIB DIMALEATE - TABLET;ORAL - GILOTRIF
From BOEHRINGER INGELHEIM; for treating locally advanced or metastatic NSCLC with non-resistant EGFR mutations or resistance to platinum-based chemotherapy

EQ 20MG BASE; EQ 30MG BASE
Approved in Jul 12, 2013, used as Reference Listed Drug
There are 3 future exclusivity(ies) for this drug product. The earliest expires on 2025-04-07, and the latest expires on 2025-10-07.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-12 | FIRST-LINE TREATMENT OF METASTATIC NON-SMALL CELL LUNG CANCER WHOSE TUMORS HAVE NON-RESISTANT EPIDERMAL GROWTH FACTOR (EGFR) MUTATIONS OTHER THAN EXON 19 DELETIONS OR EXON 21 (L858R) SUBSTITUTION MUTATIONS AS DETECTED BY AN FDA-APPROVED TEST |
EQ 40MG BASE
Approved in Jul 12, 2013, used as Reference Listed Drug and Reference Standard
There are 3 future exclusivity(ies) for this drug product. The earliest expires on 2025-04-07, and the latest expires on 2025-10-07.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-12 | FIRST-LINE TREATMENT OF METASTATIC NON-SMALL CELL LUNG CANCER WHOSE TUMORS HAVE NON-RESISTANT EPIDERMAL GROWTH FACTOR (EGFR) MUTATIONS OTHER THAN EXON 19 DELETIONS OR EXON 21 (L858R) SUBSTITUTION MUTATIONS AS DETECTED BY AN FDA-APPROVED TEST |
ARSENIC TRIOXIDE - INJECTABLE;INJECTION - TRISENOX
From CEPHALON INC; for treating refractory or relapsed acute promyelocytic leukemia in patients with prior retinoid and anthracycline chemotherapy

2MG/ML
Approved in Oct 13, 2017, used as Reference Listed Drug and Reference Standard
There are no future exclusivities for this application.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-12 | ARSENIC TRIOXIDE FOR USE IN COMBINATION WITH TRETINOIN FOR TREATMENT OF ADULTS WITH NEWLY-DIAGNOSED LOW-RISK ACUTE PROMYELOCYTIC LEUKEMIA (APL) WHOSE APL IS CHARACTERIZED BY THE PRESENCE OF THE T(15;17) TRANSLOCATION OR PML/RAR-ALPHA GENE EXPRESSION |
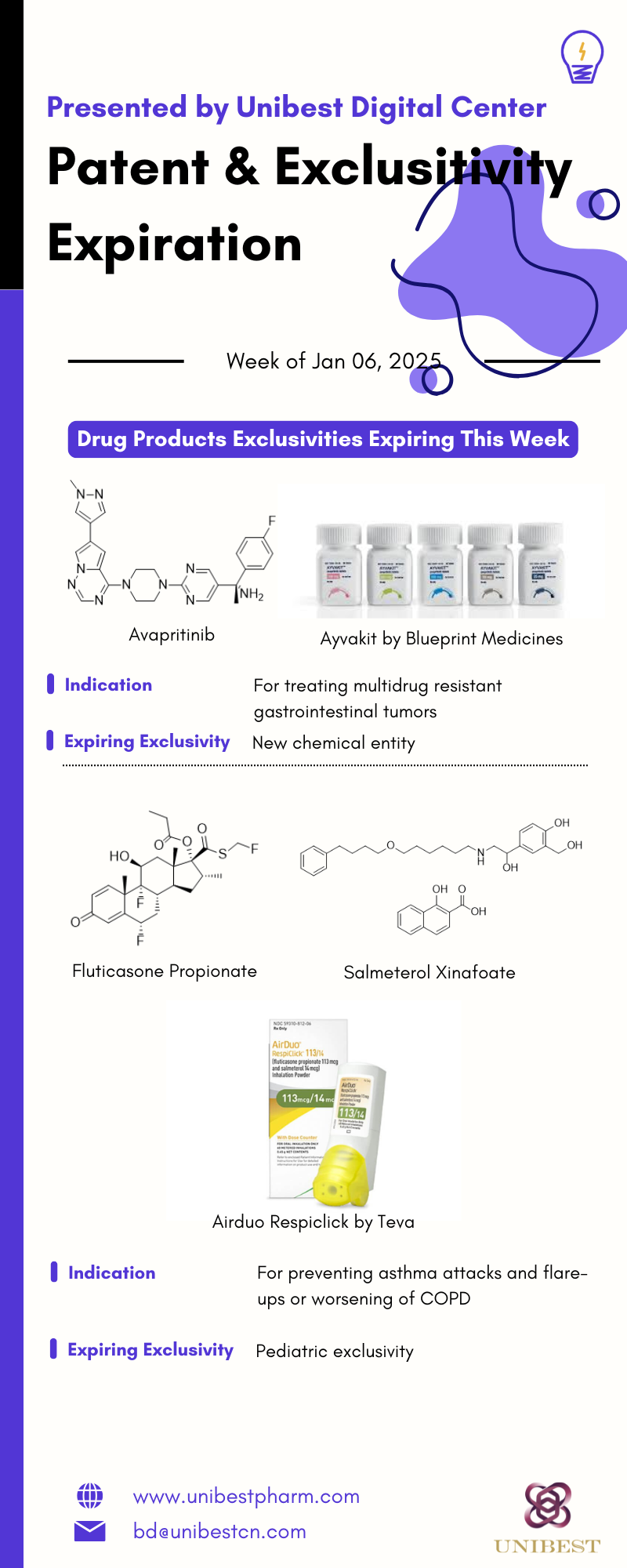
AVAPRITINIB - TABLET;ORAL - AYVAKIT
From BLUEPRINT MEDICINES CORP; for treating multidrug resistant gastrointestinal tumors

100MG; 200MG
Approved in Jan 9, 2020, used as Reference Listed Drug
There are 4 future exclusivity(ies) for this drug product. The earliest expires on 2026-05-22, and the latest expires on 2030-05-22.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-09 | NEW CHEMICAL ENTITY |
25MG; 50MG
Approved in Jun 16, 2021, used as Reference Listed Drug
There are 4 future exclusivity(ies) for this drug product. The earliest expires on 2026-05-22, and the latest expires on 2030-05-22.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-09 | NEW CHEMICAL ENTITY |
300MG
Approved in Jan 9, 2020, used as Reference Listed Drug and Reference Standard
There are 4 future exclusivity(ies) for this drug product. The earliest expires on 2026-05-22, and the latest expires on 2030-05-22.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-09 | NEW CHEMICAL ENTITY |
FLUTICASONE PROPIONATE; SALMETEROL XINAFOATE - POWDER;INHALATION - AIRDUO RESPICLICK
From TEVA PHARMACEUTICAL INDUSTRIES LTD; for preventing asthma attacks and flare-ups or worsening of COPD


0.055MG/INH;EQ 0.014MG BASE/INH
Approved in Jan 27, 2017, used as Reference Listed Drug
There are no future exclusivities for this application.
| Exclusivity Date | Exclusivity Use Definition |
| 2025-01-09 | PEDIATRIC EXCLUSIVITY |