Erythropoietin (EPO) is a growth factor naturally produced in the kidneys that stimulates the production of red blood cells. In healthy humans, one percent of all red blood cells are destroyed and replaced by reticulocytes daily. The basal production rate of 2-3×10¹¹ cells/day increases significantly when blood oxygen availability decreases. Low oxygen levels trigger erythropoietin gene expression in the kidneys and liver. In cases of severe anemia or hypoxemia, plasma EPO levels can rise up to 1,000-fold above normal.

src: The Story of Erythropoietin - Hematology.org. https://www.hematology.org/about/history/50-years/erythropoietin.
Clinical trials have demonstrated that replacement therapy with recombinant human EPO (rhEPO) effectively treats anemia in chronic renal failure by eliminating the need for blood transfusions. Furthermore, recombinant EPO shows promise in treating other types of anemia, including those associated with rheumatoid arthritis, AIDS, and malignancies.
Biochemistry of EPO
EPO is produced by liver in fetus. Kidney becomes the major site of EPO production after birth. The human EPO gene is located on chromosome 7 in the middle of its long arm in region q11-q22. It exists as a single copy and consists of five exons and four introns. The gene encodes a single polypeptide chain of 193 amino acids, which includes a 27-residue hydrophobic secretory leader sequence.
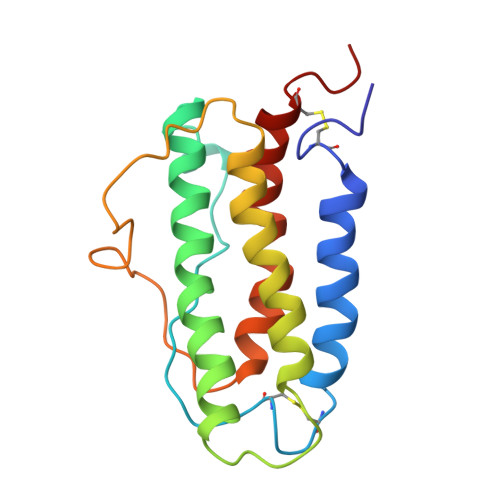
During protein synthesis, this 27-residue N-terminal leader sequence is cleaved from the 193-amino-acid gene product through cotranslational processing. The molecule then undergoes further post-translational modifications. Analysis of the C-terminus through peptide mapping and mass spectrometry has revealed that the C-terminal arginine at position 166 is absent in both human urinary and recombinant EPO. As a result, the circulating hormone contains 165 amino acids. The protein structure is stabilized by two disulfide bridges formed between cysteines at positions 7 and 161, and positions 29 and 33.

src: PDB ID 1BUY
Circular dichroism studies of EPO's secondary structure show that 50% consists of alpha-helices, while the remainder has a random configuration. The protein's globular structure is thought to form from two antiparallel pairs of alpha-helical bundles.
The EPO peptide backbone has a molecular mass of 18 kDa, while the complete glycoprotein is 30 kDa, with carbohydrates accounting for 40% of the total mass. The protein contains three N-linked glycosylation sites (Asn-24, Asn-38, and Asn-83) and one O-linked site (Ser-126) with acidic oligosaccharide chains. These chains contain fucose, mannose, N-acetylglucosamine, galactose, and N-acetylneuraminic acid. While the N-linked chains primarily form tetra-antennary complexes, the O-linked chain is shorter with just four sugar residues. The N-linked chain patterns vary between individuals. These glycosylated chains play essential roles in EPO's biological activity, protecting it from oxygen radical damage and enabling proper production and secretion of the mature hormone.

src: File:EPO.png - Wikipedia. https://commons.wikimedia.org/wiki/File:EPO.png (2006).
MOA of EPO
Blood cells are continuously generated from proliferative progenitors in the hematopoietic organs. The sequence of cell division, differentiation, and maturation involves three main developmental stages. The process begins with self-renewing pluripotent stem cells, which can produce progeny committed to erythrocytic, megakaryocytic, granulocytic, or monocytic differentiation. Under the control of various growth factors, including EPO, these cells develop into identifiable blood cell precursors.

Schema of oxygen sensing mechanism and the effect of PHD inhibitor on EPO transcription. Shih, H.-M., Wu, C.-J. & Lin, S.-L. Physiology and pathophysiology of renal erythropoietin-producing cells. Journal of the Formosan Medical Association 117, 955–963 (2018).
Like other protein hormones, EPO works by binding to specific cell membrane receptors. EPO binds to EPOR, which is expressed on colony-forming unit-erythroid (CFU-E), proerythroblasts, and early basophilic erythroblasts, preventing these progenitor cells from undergoing apoptosis. As a feedback loop, increased erythrocytes in circulation improve tissue oxygenation, which leads to decreased EPO production. Binding of EPO to EPOR triggers a conformational change in EPOR, activating Janus kinase 2 to phosphorylate tyrosine in EPOR's cytoplasmic domain. This activation initiates downstream signals, including signal transducer and activator of transcription 5, Ras, and phosphoinositol-3 kinase/AKT kinase. The EPO-EPOR binding also triggers endocytosis of the complexes, which are then degraded in lysosomes, reducing circulating EPO levels.
EPO not only stimulates the proliferation and differentiation of erythrocytic progenitors but also activates the mitotic division of proerythroblasts and basophilic erythroblasts. Additionally, it speeds up the release of reticulocytes from the bone marrow. Peak reticulocytosis typically occurs 3–4 days after a sharp increase in plasma erythropoietin.
Different Types of rhEPO
Due to the complex glycosylation process, rhEPO is primarily produced in mammalian host cells, including CHO, baby hamster kidney (BHK), and human fibrosarcoma cells (HT1080). Unlike mammalian cells, transfected bacteria such as Escherichia coli and Bacillus brevis cannot glycosylate rhEPO, while yeast and filamentous fungi perform different glycosylation modifications. CHO cells serve as the primary host cells for commercial rhEPO production in regulated markets like the US and EU.
According to WHO international nonproprietary naming conventions, EPO-type substances share a common stem ("-poetin"). When produced in eukaryotic cells with an amino acid sequence identical to human EPO, it is called "epoetin." Different prefixes (such as "darbepoetin") indicate variations in amino acid sequence. Greek letters are added to distinguish EPO types with different glycosylation patterns from various production systems.
Epoetin alfa, developed by Amgen, was the first recombinant ESA produced in CHO cells using human EPO cDNA. After its 1988 EU approval and 1989 US approval, it gained worldwide distribution under various brand names: Epogen, Procrit, Eprex, Erypo, and Espo. Though Amgen manufactures both Epogen and Procrit, Epogen is marketed by Amgen in the US specifically for dialysis patients with CKD, while Janssen-Ortho (Johnson & Johnson) markets Procrit for all other indications. Outside the US, Janssen-Cilag Ltd. and Ortho Biotech market epoetin alfa as Eprex and Erypo, while Kyowa Hakko Kirin distributes Espo in Japan.
Epoetin beta (NeoRecormon by Roche and Epogin by Chugai) is available only in non-US territories including Europe and Japan.
Epoetin delta and omega, produced in BHK cells and human HT1080 cell lines respectively, are not available in the US or EU.
The two major EPO market leaders, Amgen and Roche, have developed next-generation rhEPO molecules offering longer duration and less frequent dosing. Amgen's darbepoetin alfa (Aranesp), a hyperglycosylated rhEPO analog, received US and international regulatory approval in 2001/2002. Roche's CERA (Mircera), a PEGylated version of epoetin beta using Nektar's technology, offers monthly dosing—longer than darbepoetin alfa. While approved in both the EU and US in 2007, Mircera's sales remain limited outside the US due to Amgen's effective epoetin patent protection within the US.
Biosimilar EPOs are primarily available as Epoetin alfa, beta, or omega types and are manufactured and distributed worldwide under various brand names.
As biosimilars continue to enter the market, they provide significant cost savings for patients, physicians, insurance providers, and governments while expanding treatment options for various diseases. Studies have shown that biosimilar epoetin alfa is the most cost-effective erythropoiesis-stimulating agent for managing chemotherapy-induced anemia, using both fixed and weight-based dosing strategies. With fixed-dosing scenarios, biosimilar rhEPO alfa 40,000 IU yields average cost savings of €990 (13.8%) to €3,042 (33.0%), while the 30,000 IU version saves €2,534 (35.4%) to €4,587 (49.7%). Weight-based dosing with biosimilar rhEPO alfa provides cost savings ranging from €757 (13.8%) to €3,738 (44.2%).
Our Offering
Unibest is proud to partner with a renowned Chinese pharmaceutical company to provide epoetin alfa (produced in CHO cells) for both drug substance and drug product collaborations. Our epoetin alfa, a National Class 2 New Drug launched in 1998, is formulated as a small-volume injection. It primarily treats anemia caused by renal insufficiency in both dialysis and non-dialysis patients, and supports perioperative red blood cell mobilization in surgery. The product shows excellent tolerability with efficacy and safety comparable to imported alternatives. This product was included in the "Ninth Five-Year" National Science and Technology Tackling Plan and was recognized among Shandong Province's "Top 10" High-Tech Products and Innovation Plan projects. Having achieved internationally advanced and domestically leading standards, it is available in six specifications: 2000IU/1ml/vial, 3000IU/1ml/vial, 4000IU/1ml/vial, 5000IU/1ml/vial, 9000IU/1ml/vial, and 12000IU/1ml/vial.

References
2. Jelkmann, W. Erythropoietin: structure, control of production, and function. Physiological Reviews 72, 449–489 (1992).
3. Maiese, K., Li, F. & Chong, Z. Z. New Avenues of Exploration for Erythropoietin. JAMA 293, 90–95 (2005).
5. Lee, J. S., Ha, T. K., Lee, S. J. & Lee, G. M. Current state and perspectives on erythropoietin production. Appl Microbiol Biotechnol 95, 1405–1416 (2012).
6. Ebied, W. M., Ahmed, H. M. & Elbarbry, F. A. Production and analysis of a biosimilar erythropoietin in Egypt. Biosimilars 4, 11–22 (2014).
7. Bocquet, F. et al. Biosimilar Versus Patented Erythropoietins: Learning from 5 Years of European and Japanese Experience. Appl Health Econ Health Policy 13, 47–59 (2015).
8. Goldsmith, D., Dellanna, F., Schiestl, M., Krendyukov, A. & Combe, C. Epoetin Biosimilars in the Treatment of Renal Anemia: What Have We Learned from a Decade of European Experience? Clin Drug Investig 38, 481–490 (2018).
9. Shih, H.-M., Wu, C.-J. & Lin, S.-L. Physiology and pathophysiology of renal erythropoietin-producing cells. Journal of the Formosan Medical Association 117, 955–963 (2018).
10. Anand, S., Al-Mondhiry, J., Fischer, K. & Glaspy, J. Epoetin alfa-epbx: a new entrant into a crowded market. a historical review of the role of erythropoietin stimulating agents and the development of the first epoetin biosimilar in the United States. Expert Review of Clinical Pharmacology 14, 1–8 (2021).