By Stephanie Gaulding, managing director, Pharmatech Associates—a USP company
Building strong relationships with chosen outsourcing partners, namely contract development and manufacturing organizations (CDMOs) and contract research organizations (CROs), relies on a solid outsourcing framework. Part of this framework centers on ensuring that drug sponsors provide the necessary oversight of their outsourcing partners, across the product development cycle. The ideal operating model for these engagements is one where the drug sponsor calibrates its oversight to the specific nuances of its CDMO and CRO partners according to the details of each relationship. Defining criteria ahead of time minimizes surprises, allows for fluid communication between the entities, and results in optimum performance. The aim of this planning process is quick and accurate response. We see three key areas that should be built into your outsourcing framework and agreements.

1.Oversight Plans
Oversight plans are a tool borne from overseeing outsourced clinical activities that can be leveraged into a broader spectrum of outsourcing relationships. In practice, such plans define the framework of a specific engagement with an outsourcing partner and allow a drug sponsor to adjust their oversight plans based on identified risks, partner performance, and the overall nature of the project.
An oversight plan is typically project- or engagement-specific because the people involved can vary. Adjustments can be made in response to newly identified risks, changes in approaches to mitigating risks, or changes in the capabilities of the outsourcing partner. Oversight plans have four core components:
General Information
This section describes the overall nature of the specific engagement between a drug sponsor and an outsourcing partner and summarizes the project’s boundaries, including if additional entities or partners are involved in completing the engagement. This section typically changes only when a change in the overall purpose or scope of the engagement with an outsourcing partner occurs.
Interactions Between Entities
This section details the overall governance for the engagement, including identifying primary and routine points of contact, defining the escalation process for both technical and quality issues arising during the engagement, and describing the communication plans for the engagement. In crafting this section, it is essential to be as specific as possible in the names of individuals, their preferred method of communication, and desired frequency of communication. This section should also include the meeting structure and cadence necessary to ensure the drug sponsor maintains appropriate oversight. The information contained in this section requires periodic updates as people or communication methods change and provides one of the adjustment levers to increase or decrease oversight of an outsourcing partner.
Performance Criteria
This section defines the specific metrics and key performance indicators (KPIs) that will be monitored as part of overseeing the defined engagement and includes methods and frequency for sharing updated performance results between the drug sponsor and the outsourcing partner. Drug sponsors should be thoughtful about setting metrics and KPIs that will be meaningful in assessing not only the performance of the supplier but also the health of the relationship. Like any robust performance monitoring program, a significant change in the capabilities of the outsourcing partner or any newly identified risks should trigger a review and adjustment, if necessary, of the performance criteria.
Data, Risk, And Knowledge Management
This section can be a single integrated section or be arranged into discrete sections addressing each topic. In building these areas of the oversight plan, focus on the mechanisms and processes, documenting the tools to support each of these areas and detailed methods or procedures that describe the interactions between the two (or more) entities. Of course, referencing supporting documents is recommended if they are formalized and available to both parties.
It does neither party any good to expend energy developing an oversight plan just to “put it on a shelf.” Oversight plans need to be readily available to both parties throughout the engagement. They must be arranged so that the original approach can be reviewed and adjusted in response to triggers such as risks, changes in personnel, etc.
2. Risk Management
Achieving calibrated oversight of outsourcing partners relies heavily on robust risk management to evaluate emerging business, quality, and regulatory compliance signals. When establishing a new engagement with an outsourcing partner, drug sponsors should conduct baseline reviews of a partner’s systems, processes, infrastructure, etc., to identify risks that need to be monitored or mitigated. On the surface, these reviews may seem redundant with activities performed during the due diligence and selection process. Still, if done right, they build on those initial partner assessments and dive deeper into the mechanics of how the engagement will work (versus how the engagement might work). Risks identified in these baseline assessments can drive mitigating controls or monitoring through a risk review process.
Either way, establishing a feedback loop between the various risk assessments, their associated risk controls, and the oversight plan allows a drug sponsor to modulate oversight requirements (e.g., frequency of review meetings, metrics, KPIs). This feedback loop supports a more robust monitoring process, especially where significant risks exist without sufficient controls or mitigation measures.
Of course, no conversation about risk management is complete without discussing risk review and communication. Structured periodic reviews of the initial baseline assessments need to be done in a manner that challenges the assumptions made are still valid, ensures incorporation of new information into the risk assessments, and verifies planned controls and mitigation measures are working as intended. Without these periodic reviews, risks grow stale and out of touch with the current state of the engagement, and there can be little consideration for potential future disruptions.
3. Change Management
Change management is central in a drug sponsor’s relationship with their outsourcing partners, as the drug sponsor holds the ultimate accountability and responsibility for compliance with an approved marketing authorization. This is one of the responsibilities that cannot be delegated to another party; therefore, it is essential that the process for managing changes that impact commitments made to health authorities by the drug sponsor provides the drug sponsor with timely notification by the outsourcing partner.
It is not sufficient to rely solely on individual entity-specific change management processes. Instead, consider building a process map specific to the relationship between the drug sponsor and outsourcing partner and integrating this into the oversight plan or quality agreement. From the drug sponsor’s viewpoint, it needs to have visibility of changes across its supply chains, meaning that a proposed change coming from one outsourcing partner may have ramifications up, down, or across the drug sponsor’s overall supply chain. On the drug sponsor side, transparency in how each process works creates better visibility into situations that can cause a change cascade and leads to better communication and decision-making.
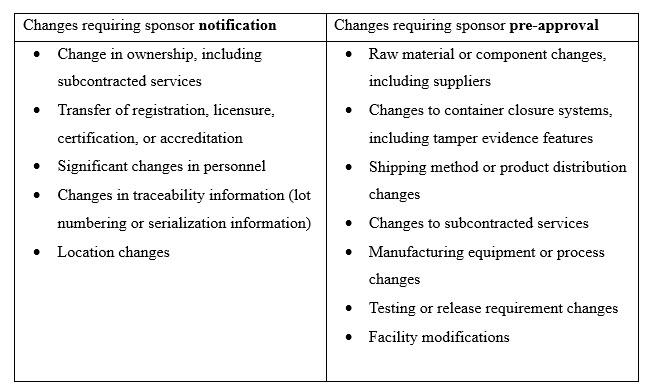
Additionally, not all changes by outsourcing partners require the same type of action by the drug sponsor. Some changes require that a drug sponsor is appropriately notified of a change so it can update the approved marketing authorization; other changes require a drug sponsor’s review and approval prior to implementation, allowing the drug sponsor to assess the impact to an approved marketing authorization and the potential for triggering a change cascade. Table 1 provides examples of changes in each category of drug sponsor action.
Table 1. Examples of changes based on drug sponsor action type.

One Final Thought
Appropriate oversight is key to success with outsourcing partners, whether involved in the stages of development, manufacturing, or clinical trial research. Minimizing exceptions to your partners’ systems and processes lowers the overall risk of the drug sponsor and its CDMO or CRO falling out of compliance with their approved marketing authorization. In short, it helps build stronger relationships between partners. To achieve this vision, it’s important to tailor oversight specific to each outsourcing partner. This not only benefits the drug sponsor, but it also allows them to focus attention proactively across their overall supply chain and to move away from reactive partnerships.
About The Author:

Stephanie Gaulding, Pharmatech Associates’ managing director, has more than 25 years of experience in the pharma, biotech, medical device, and related life science industries developing and delivering sustainable quality management systems that assure compliance with global regulatory requirements and industry best practices. She is an ASQ-certified quality auditor and ASQ-certified pharmaceutical GMP professional. She holds a M.Sc. in biotechnology from Johns Hopkins University and a B.Sc. in biology from Virginia Tech.