After the failure of several NASH drugs from Novartis, Boehringer Ingelheim, Genfit, Gilead, Albireo and NGM, the field of nonalcoholic steatohepatitis (NASH) finally ushered in positive progress. Intercept announced the latest data from the pivotal Phase 3 REGENERATE study of its representative drug obeticholic acid (OCA).
This is the second analysis of the efficacy of OCA in patients with NASH-induced liver fibrosis. According to the regulatory guidance for NASH R&D issued by the FDA, the results of this interim analysis using a consensus panel approach show that this The probability of approval of this product is further increased. As soon as the news came out, the stock prices of NASH-related R&D companies in the U.S. stock market rose across the board, and the market seemed to regain some optimism about the listing prospects of NASH products.
A study published in the "New England Journal of Medicine" shows that advanced fatty liver has replaced hepatitis C as the leading cause of liver scarring and liver transplantation (nearly 7-fold increased risk of death), and there is currently no specific drug for NASH on the market. , there is a huge gap in clinical satisfaction. Does the updated results of this study mean that the FXR target will finally bottom out and enter the bottomless blue ocean market of NASH first...
Significantly undervalued NASH market size
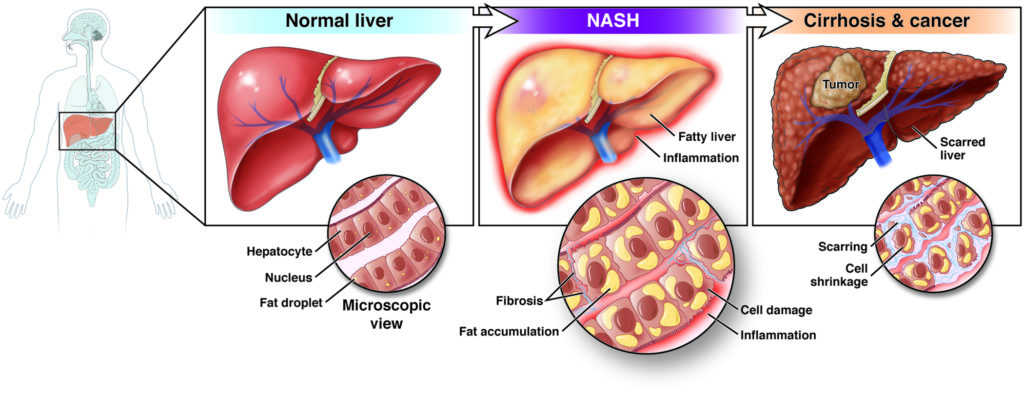
Not all fatty liver disease is called NASH, and not all hepatitis is classified as NASH. Fatty liver, inflammation, and liver cell damage must be present to be diagnosed as NASH (non-alcoholic steatohepatitis).
NASH is MAFLD with further deterioration of liver function. When the liver accumulates too much fat that cannot be consumed, liver cells can only work overtime to "sudden death". At this time, the body will activate the immune response through an inflammatory response to "cut off" necrotic cells. When a sufficient number of new employees (regenerating liver cells) cannot be recruited in time, the holes in the liver can only be filled with "fishing" fibrous tissue. In this way, under the condition that the fat content remains the same or only increases, the fewer and fewer liver cells have to work overtime, and then start the cycle of negative compensatory mechanism (fibrous tissue filling) until Progress to decompensated cirrhosis (depletion of liver function) or development of hepatocellular carcinoma.

Non-alcoholic fatty liver disease (MAFLD) has gradually become one of the chronic liver diseases with the highest incidence in the world. The prevalence of MAFLD in adults worldwide is about 15%-30%, of which at least 15%-25% of patients have disease progression. for NASH. Epidemiological data show that the total number of people with NAFLD in the United States can reach 80 million, of which about 15 million are diagnosed as NASH patients. When it comes to the "disease of wealth", China is naturally unwilling to follow. In the past 10 years, the prevalence of NAFLD in adults (diagnosed by ordinary B-mode ultrasound) has increased from 15% to 31%. It is estimated that there are tens of millions of NASH patients. Moreover, limited by various factors such as disease characteristics and scientific research technology, the patient base we see may only be the tip of the iceberg...
Hidden disease group & huge potential group
Patient hospital visits were lower. Because the liver has a strong compensatory ability and has almost no pain nerves, it is difficult for the human body to send out a distress signal. Therefore, NASH is called a "silent chronic disease". Before it develops to liver cirrhosis, there will be no clinical symptoms that seriously affect the quality of life. As a result, it is difficult for patients to detect abnormal lesions in an early stage, and the hospital visits of patients are low, and there is a lack of motivation for interventional treatment for NASH.
The second is the difficulty of diagnosis. Compared with other major diseases (high blood pressure can be measured directly, hyperlipidemia and diabetes can be judged by blood test), the common detection method of NASH, which is also a chronic disease, has some bugs:
Blood test: the most accessible to patients, however, the accuracy of common indicators of liver injury (including several liver enzymes including alanine aminotransferase) is limited, and various random factors such as staying up late, dancing and trauma can cause it fluctuations.
Imaging examination methods: low-cost traditional B-ultrasound can only detect symptoms in the middle and late stages, while the popular Fibroscan abroad cannot detect liver fibrosis and inflammation. More accurate MRI and enhanced CT are expensive!
Tissue biopsy: Puncture sampling, microscopic observation of the lesion, which is the only gold standard designated by the FDA. However, invasive procedures can cause physical wounds and are expensive in time and money!
Obesity, type II diabetes, hyperlipidemia and hypertension are regarded as the main risk factors for MAFLD. The incidence of MAFLD in obese people is as high as 70%. With the continuous increase of obesity and the three high population, the prevalence of MAFLD will continue to rise trend. After the hepatitis C was cleaned up, the "rich disease" NASH is the one with the most rapid growth and the most attention in the field of liver disease. After all, globally, NASH has become the second most common cause of liver transplantation after hepatitis C.
As large-scale chronic diseases such as blood pressure, blood lipids, and blood sugar are gradually conquered, and they are gradually falling into the Red Sea, NASH, which has not yet been approved for marketing in the world, has become the only remaining "big cake" in the eyes of various pharmaceutical companies. According to Evaluate Pharm and Frost & Sullivan, the global market for NASH drugs will reach US$35-40 billion in 2025.
Drug candidates are combed across the board
The 31st Annual Meeting of the Asia-Pacific Society of Liver Diseases (APASL 2022) made a special report on the replacement of the existing name "Non-alcoholic fatty liver disease (NAFLD)" by "Metabolic-Associated Fatty Liver Disease (MAFLD)". In the era of diagnosis and treatment, NASH/NAFLD is essentially a metabolic disorder involving various factors such as glucose and lipid metabolism.
Bringing a new perspective, the research ideas of related drugs have also changed from focusing directly on the improvement of fibrosis to focusing on the efficacy indicators related to the pathophysiological characteristics of MAFLD (control of inflammation and reduction of fat accumulation, etc.). Most of the drugs that go straight to anti-fibrosis have fallen one after another in the clinical stage, which also proves this: although anti-fibrosis is the clinical need most closely related to the benefit of patients, the NASH treatment process is similar to "thrombolysis" , the underlying logic (inflammation and fat) does not work, and it still does not work.

The huge market structure that is like a blank sheet of paper is in front of us, which naturally attracts various types of pharmaceutical companies. In the past two years, with the deepening of NASH mechanism research, many innovative NASH drugs in the above picture have been successively advanced to the clinical stage, mainly focusing on 3 key factors affecting the disease process - steatosis, inflammation and fibrosis.
Drug candidates are combed across the board
Compared with other mainstream targets such as FXR, THR-β, FGF19/21, and GLP-1, FXR has clinically prespecified endpoints (liver drug enzymes, reduction in relative liver fat content, NAS scores, and serum biomarkers) Agonists have obtained the most reliable clinical efficacy data at present. After all, with the current sample size, it is difficult for the above non-invasive diagnostic methods (NIT, non-invasive test) to establish a strong correlation with all-cause mortality or disease progression (note: not with biopsy!).
In addition, the data on the mechanism of action of THR-β is also very beautiful at first glance, and the lipid-lowering energy is very strong, but fatty liver is not a gap in clinical demand, and hunger can really relieve or even cure, and the efficacy of the product is similar to that of NASH. The core element—inflammation—was weaker.
But then again, the current clinical data for FXR agonists can only barely qualify as a "general of dwarfs." Of course, this does not depend entirely on the defects of the target itself, but also involves the gold standard (biopsy) in this field of disease.
Ultimately, the success of the above mainstream targets in clinical use largely depends on the safety of the product (itching, weight gain, vomiting and diarrhea, etc.) and the final patient group positioning (fatty liver or NASH).
Patients with liver biopsy-proven NASH fibrosis were randomized 1:1:1 to receive placebo (n=311), OCA 10 mg (n=312), or OCA 25 mg (n=308) once daily. Subjects were biopsied again at month 18 for an interim analysis.
The new analysis uses a new consensus radiograph (biopsy result) method for reassessment of liver biopsies at baseline and at month 18. So far, of the more than 2,000 patients who have been studied, nearly 1,000 patients have been taking medication for four years. The following table lists the data of the first analysis and the data of the re-analysis.
Patients with liver biopsy-proven NASH fibrosis were randomized 1:1:1 to receive placebo (n=311), OCA 10 mg (n=312), or OCA 25 mg (n=308) once daily. Subjects were biopsied again at month 18 for an interim analysis.
The new analysis uses a new consensus radiograph (biopsy result) method for reassessment of liver biopsies at baseline and at month 18. So far, of the more than 2,000 patients who have been studied, nearly 1,000 patients have been taking medication for four years. The following table lists the data of the first analysis and the data of the re-analysis.

For the quality-adjusted life years (QALY) achieved by drug treatment, countries or regions with different economic levels have different payment standards. In the United States, the willingness to pay for each quality-adjusted life year (ICER value, incremental cost-effectiveness ratio, meaning incremental cost-effectiveness ratio) is usually around US$50,000, which is less than one per capita GDP. For serious diseases, this value can be used. Widen the range. According to the evaluation results and the patient benefits achieved in the clinical practice of obeticholic acid, the pricing under different payment standards is shown in the table below.

Reference:
1. Lancet. 2019 Dec 14;394(10215):2184-2196. doi: 10.1016/S0140-6736(19)33041-7. Epub 2019 Dec 5. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial.
2. Evidence Report--Obeticholic Acid for the Treatment of Nonalcoholic Steatohepatitis with Fibrosis.
3. Cell. 2021 May 13;184(10):2537-2564. doi: 10.1016/j.cell.2021.04.015. Mechanisms and disease consequences of nonalcoholic fatty liver disease.
4. Nat Rev Gastroenterol Hepatol. 2021 Jun;18(6):373-392. doi: 10.1038/s41575-020-00408-y. Therapeutic pipeline in nonalcoholic steatohepatitis.